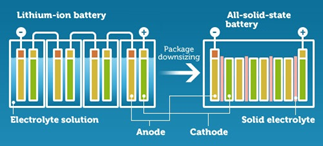
Solid State Batteries Are Getting Real Attention These Days
Benefits of solid-state batteries over Lithium-ion:
- Safer (No explosion or catching Fire!!)
- Longer life spans
- Smaller in size- (Li-ion: Separators come in 20-30 micron thickness. Solid-state technology can decrease the separators down to 3-4 microns each).
- Highly Conductive (Less internal resistance, hence higher efficiency)
Has lithium-battery genius John Goodenough done it again?

Google’s Eric Schmidt Tweet
- Goodenough reported that his new battery cell had achieved a 10-fold improvement in energy density in one case, and a three-fold improvement in another.
- In one experiment, Goodenough estimates a 30-fold improvement on the best density in a lithium-ion battery today—8,500 watt-hours per kilogram.
- Moreover, this was accomplished not using exotic materials, but cheap sodium and sulfur. That means, unlike many other reported battery breakthroughs, this one could actually be used in mainstream-priced cars.
http://pubs.rsc.org/en/Content/ArticleLanding/2017/EE/C6EE02888H#!divAbstract
HALLOYSITE NANOTUBEs:
A research group led by Professor Jan D. Miller of the University of Utah’s Department of Metallurgical Engineering has received a $191,700 grant to aid the development and commercialization of a solid polymer electrolyte/electrode technology for lithium batteries
Highlights:
- Solid-state batteries generally fall into the low-power density and high-energy density category
- Natural halloysite nanotubes (HNT) are directly used as solid electrolyte filler.
- Oppositely charged HNT surfaces enhance lithium ionic conductivity.
- Application of the HNT electrolyte is demonstrated by high energy Li-S battery.
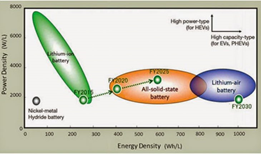
Fig: Power Density vs Energy Density comparison chart
Silicon Anodes replaces conventional Graphite Anodes, would it optimize the performance?
Silicon anodes are receiving a great deal of attention from the battery community. They can deliver around three to five times higher capacity compared with those using current graphite anodes in lithium ion batteries.
Silicon has been shown to have a high theoretical gravimetric capacity, approximately 4200 mA h/g, compared to only 372 mA h/g for graphite.
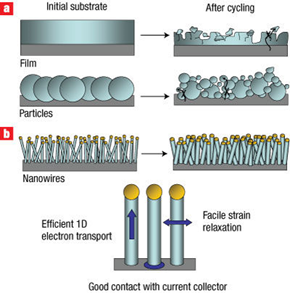
- A KAIST research team led by Professors Jang Wook Choi and Ali Coskun reported a molecular pulley binder for high-capacity silicon anodes of lithium ion batteries in Science on July 20, 2017.
- The KAIST team integrated molecular pulleys, called polyrotaxanes, into a battery electrode binder, a polymer included in battery electrodes to attach the electrodes onto metallic substrates. In a polyrotaxane, rings are threaded into a polymer backbone and can freely move along the backbone.
Zinc-Air batteries - Sounds promising!
University of Sydney researchers have found a solution for one of the biggest stumbling blocks preventing zinc-air batteries from overtaking conventional lithium-ion batteries as the power source of choice in electronic devices.
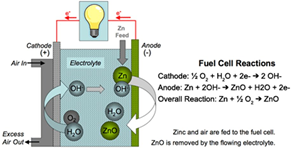
Zinc-air batteries are batteries powered by zinc metal and oxygen from the air. Due to the global abundance of zinc metal, these batteries are much cheaper to produce than lithium-ion batteries, and they can also store more energy (theoretically five times more than that of lithium-ion batteries), are much safer and are more environmentally friendly.
These new catalysts are produced through the simultaneous control of the:
1) Composition,
2) Size and
3) Crystallinity of metal oxides of earth-abundant elements such as iron, cobalt and nickel. They can then be applied to build rechargeable zinc-air batteries.
The trials of zinc-air batteries developed with the new catalysts had demonstrated excellent rechargeability -- including less than a 10 percent battery efficacy drop over 60 discharging/charging cycles of 120 hours.


