Beyond the Lithium-ion battery! A Look at Supercapacitors And Other Batteries
Supercapacitors
A supercapacitor (also electric double-layer capacitor (EDLC), also called supercar, ultra capacitor or Goldcap) is a high-capacity capacitor with capacitance values much higher than other capacitors (but lower voltage limits) that bridge the gap between electrolytic capacitors and rechargeable batteries.
|
|
SuperCapacitor |
Lithium-ion |
|
Charge Time |
1-20 seconds |
10-60 minutes |
|
Cycle life |
1 million |
>500 |
|
Cell Voltage |
2.3 -2.7V |
3.6V (nominal) |
|
Specific energy |
5 Wh/kg |
1000-3000 Wh/kg |
|
Service life |
10-15 years |
5-10years |
|
Cost |
10,000 USD |
250-1000 |
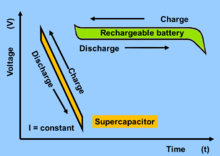
Fig: Voltage behavior of lithium ion battery and supercapacitor
Advantages:
Virtually unlimited cycle life
High specific power
Low resistance
Enables high load currents
Charges in seconds
no end-of-charge termination required
Simple charging, draws only what it needs, not subject to overcharge
Safer
Excellent low-temperature charge and discharge performance
Disadvantages:
Linear discharge voltage prevents using the full energy spectrum
High self-discharge; higher than most batteries
Low cell voltage; requires series connections with voltage balancing
High cost per watt
Fact: As of 2013 commercially available lithium-ion supercapacitors offered the highest gravimetric specific energy to date, reaching 15 Wh/kg (54 kJ/kg)
Lithium-Air
Anode-Cathode: Lithium- Porous carbon (Oxygen)
Advantages: 10x greater energy density than Li-ion
Disadvantages: Air is not pure enough and would need to be filtered. Lithium and oxygen form peroxide films that produce a barrier, ultimately killing storage capacity. Cycle life is only 50 cycles in lab tests.
Variations: Scientists also trying aluminum-air and sodium-air batteries as well.
Lithium-Sulphur
Anode- Cathode: Lithium- Sulphur, Carbon
Advantages: Lighter, cheaper, and more powerful than li-ion
Disadvantages: Volume expansion of up to 80%, causing mechanical stress. Unwanted reactions with electrolytes. Poor conductivity and poor stability at higher temperatures.
Variations: Many different variations exist, including using graphite/graphene, and silicon in the chemistry.
Vanadium Flow Batteries
Anolyte - Catholyte: Vanadium -Vanadium
Advantages: Using vanadium ions in different oxidation states to store chemical potential energy at scale. Can be expanded simply by using larger electrolyte tanks.
Disadvantages: Poor energy-to-volume ratio. Very heavy; must be used in stationary applications.
Variations: Scientists are experimenting with other flow battery chemistries as well, such as zinc-bromine.
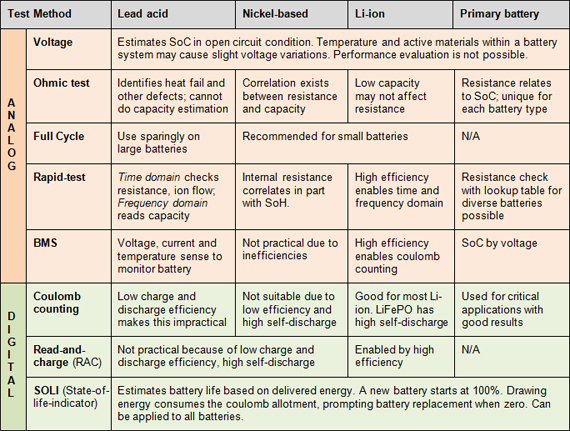
Fig: Battery test methods for common battery chemistries
Courtesy: Batteryuniverity.com
TIP: Charge Li-ion battery at a moderate rate. Ultra-fast charging works only to 70 percent state-of-charge (SoC) and causes stress; topping charge takes longer (NiCad is the only battery that can accept ultra-fast charge with minimal stress.)


